In physics, radiation is a process in which energetic particles or energetic waves
travel through a vacuum, or through matter-containing media that are not required
for their propagation. Waves of a mass filled medium itself, such as water
waves or sound waves, are usually not considered to be forms of
"radiation" in this sense.
The word radiation is often colloquially used in
reference to ionizing radiation (e.g. x-rays, gamma rays), but the term radiation
may correctly also refer to non-ionizing radiation (e.g., radio waves, heat or visible light) as well. The particles
or waves radiate (i.e.,
travel outward in all directions) from a source. This aspect leads to a system
of measurements and physical
units that are applicable to all
types of radiation. Because radiation expands as it passes through space, and
as its energy is conserved (in vacuum), the power of all types of radiation
follows an inverse-square law in relation to the distance from its
source.
Both ionizing and non-ionizing radiation can be harmful to organisms and can result in changes to the natural environment. In general,
however, ionizing radiation is far more harmful to living organisms per unit of
energy deposited than non-ionizing radiation, since the ions that are produced,
even at low radiation powers, have the potential to cause DNA damage. By
contrast, most non-ionizing radiation is harmful to organisms only in
proportion to the thermal energy deposited, and is conventionally considered
harmless at low powers that do not produce a significant temperature rise. Ultraviolet radiation in some aspects occupies a
middle ground, as it has some features of both ionizing and non-ionizing
radiation. Although nearly the entire ultraviolet spectrum that penetrates the
Earth's atmosphere is non-ionizing, this radiation does far more damage to many
molecules in biological systems than can be accounted for by heating effects
(an example is sunburn). These
properties derive from ultraviolet's power to alter chemical bonds, even
without having quite enough energy to ionize atoms.
Electromagnetic Radiation
Electromagnetic radiation (EMR) is represented as
self-propagating waves. EMR has electric and magnetic field components that
oscillate in phase perpendicular to each other and also to the direction of
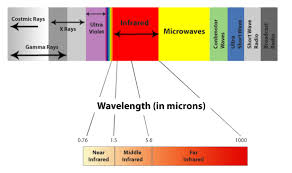
energy propagation. EMR is classified into types according to the frequency
range of the waves, these types include (in order of increasing frequency): radio
waves, microwaves, infrared radiation, visible light, ultraviolet
radiation, X-rays and gamma rays. Of these, radio waves have
the longest wavelengths (lowest energy) and gamma rays have the shortest and
hence the highest energy. A small window of frequencies, called the visible spectrum or light,
is sensed by the eyes of various organisms.

Radio Waves - are a type of electromagnetic radiation with wavelengths in the electromagnetic spectrum longer than infrared light. Like all other electromagnetic waves, they travel at the speed of light. Naturally occurring radio waves are made by lightning, or by certain astronomical objects. Artificially generated radio waves are used for fixed and mobile radio communication, broadcasting, radar and other navigation systems, satellite communication, computer networks and innumerable other applications.
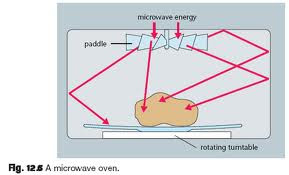
Microwaves - are electromagnetic waves
with wavelengths ranm as short as one millimeter to as long as one
meter, which equates to a frequency range of 300 GHz to 300 MHz .
Infrared - light is electromagnetic radiation with longer wavelengths than those of visible light, extending from
the nominal red edge of the visible spectrum at 700 nanometers (nm) to 1 mm. This range of wavelengths corresponds to a frequency range of approximately
430 THz down to 300 GHz, and includes most of the thermal radiation emitted by objects near room
temperature. Infrared light is emitted or absorbed by molecules when they change their rotational-vibrational movements.
 Visible light (commonly referred to simply as light) - is electromagnetic radiation that is visible to the human eye, and is responsible
for the sense of sight. Visible light has a wavelength in the range of about 380 nanometers (nm), or 380×10−9 m,
to about 740 nanometers – between the invisible infrared, with longer
wavelengths and the invisible ultraviolet, with shorter wavelengths.
Visible light (commonly referred to simply as light) - is electromagnetic radiation that is visible to the human eye, and is responsible
for the sense of sight. Visible light has a wavelength in the range of about 380 nanometers (nm), or 380×10−9 m,
to about 740 nanometers – between the invisible infrared, with longer
wavelengths and the invisible ultraviolet, with shorter wavelengths.
Ultraviolet (UV) light - is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, that is, in the range between
400 nm and 10 nm,
corresponding to photon energies from 3 eV to 124 eV. It is so-named because
the spectrum consists of electromagnetic waves with frequencies higher than
those that humans identify as the color violet. These frequencies are invisible
to humans, but visible to a number of insects and birds.
UV light is found in sunlight (where it constitutes about 10% of the energy in vacuum) and is emitted by electric arcs and specialized lights such as mercury lamps and black lights. It can cause chemical reactions, and causes m

X-radiation (composed of X-rays) - is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30exahertz (3×1016 Hz to 3×1019 Hz) and energies in the range 100 eV to 100 keV. The wavelengths are shorter than those of UV rays and longer than those of gamma rays. In many languages, X-radiation is called Röntgen radiation, after Wilhelm Röntgen, who is usually credited as its discoverer, and who had named it X-radiation to signify an unknown type of radiation.

Gamma radiation, also known as gamma rays, - refers to electromagnetic radiation of high frequency and therefore high energy per photon. Gamma rays are ionizing radiation, and are thus biologically hazardous. They are classically produced by the decay from high energy states of atomic nuclei (gamma decay), but are also created by other processes. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium. Villard's radiation was named "gamma rays" by Ernest Rutherford in 1903.
Natural sources of gamma rays on Earth include gamma decay from
naturally occurring radioisotopes,
and secondary radiation from atmospheric interactions with cosmic ray particles. Rare terrestrial natural
sources produce gamma rays that are not of a nuclear origin, such as lightning strikes and terrestrial gamma-ray flashes.